

In their research, the researchers aim to generalize Boltzmann’s simple equation identifying mathematical relationships which build up to the overall logarithmic relationship.Īll microscopic changes in a complicated closed system conspire in a way that the total entropy never decreases. Yet although the formula is so effective in mathematically predicting changes in entropy, Dr Biró and colleagues believe it cannot completely account for the more complex physical processes which underlay it. He proposed that its value simply has a logarithmic relationship with the number of possible states a system can have under its current conditions. In the 1870s, Austrian physicist Ludwig Boltzmann was able to reduce the quantity of entropy down to a remarkably simple, elegant formula. However, in their research, Dr Biró and his colleagues now want to take the theory a step further. All random, or ‘stochastic’ models are now checked for satisfying such a theorem, accounting for random forces in their calculations.” Even after centuries of scrutiny, these mathematical descriptions have proved time and again to robustly describe real physical systems. “They can be viewed as given mathematical formulae based on the probability of being in a given state. “Kinetic theory and its follower, statistical physics, depend on our knowledge about entropy,” Dr Biró continues. To quantify entropy, physicists ultimately need to use statistics to study how particles move around into different arrangements over time. All microscopic changes in a complicated closed system conspire in a way that the total entropy never decreases.” “Many processes in nature end up only in some selected states while they may begin in any of a vastly larger amount of possible states.
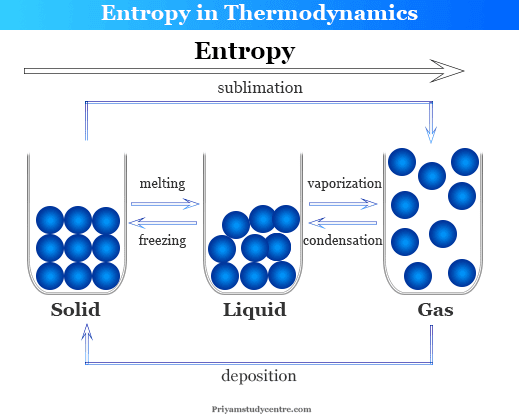
“Macroscopically, entropy has proved never to decrease spontaneously an idea related to the ‘arrow of time’, or irreversibility,” he explains.
The law states that in any system closed off from the outside, entropy must not decrease over time as such, the universe becomes more chaotic as a whole.Īs Dr Biró describes, this inevitable increase in entropy represents a decrease in the number of possible leftover arrangements of particles, or ‘states’, that a system can find itself in as the one-way passage of time progresses. The changes in entropy which take place during physical processes form the basis of one of the most fundamental laws of physics: The Second Law of Thermodynamics. Since the 19th century, physicists have described this chaos using a quantity known as ‘entropy’. While atoms within metals are arranged in neat, highly ordered lattices, the movements of gas molecules in the air are far more chaotic and unpredictable. Through constructing ‘master equations’ to describe these relationships, Biró and his colleagues are using statistics to study problems as diverse as the formation of hadrons, changes in biodiversity, and patterns in popularity on Facebook.Įvery system around us has a different degree of chaos associated with it. Yet however elegant, Tamás Biró at the Hungarian Academy of Science believes the formula is hiding a more complex array of relationships. Now we obtain two important equations from above derived expressions, namely:īoth equations are known as Gibbs Equation.Entropy is one of the most important and most widely studied quantities in physics, and for centuries, its value has been robustly described using simple mathematical relationships. We can rewrite the equation of entropy by using the 1.Law of thermodynamics: We write down here the definition of entropy:Īccording to the 1.Law of thermodynamics for reversible process which is expressed as:
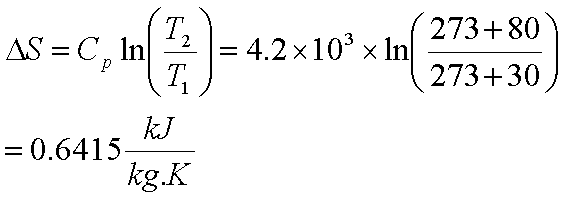
So if we want to calculate the entropy change ΔS, we can arbitrarily choose some imaginary reversible processes from initial state to final state. Recall that entropy, which is defined as δq rev/T, is a property of state.
#Entropy equation download#
Download the script: Entropy change of ideal gases


 0 kommentar(er)
0 kommentar(er)
